Exercise and Genetics Reboot
03/14/1717:37
When I wrote the original “Exercise and Genetic Variability" presentation / lecture for the 2006 High Intensity Training Seminar, hosted by Bo Raily in Indianapolis, the information presented was ground breaking.
When I wrote the original “Exercise and Genetic Variability" presentation / lecture for the 2006 High Intensity Training Seminar, hosted by Bo Raily in Indianapolis, the information presented was ground breaking. It wasn't ground breaking in that we didn’t know or suspect that genetics heavily influence our results from exercise, but that science was starting to discover the exact genes responsible for the wide variability that exist in our ability to tolerate and adapt to an exercise stimulus. A few genetic mutations had previously been discovered, for example, myostatin deletion, local IGF-I splice variant over expression, as well as a few others.
Many of you may be familiar with the Belgium Blue Bull, which was simply bred for muscularity, leading to a mutation for myostatin deletion (fig.1) These bulls were not put on any special training programs, nutritional supplements, creatine monohydrate or hydrochloride, protein powders, anabolic steroids, etc. They simply stand around and graze like any other cow. Myostatin deletion leads to a virtual doubling of the number of muscle fibers when compared to animals without myostatin deletion.
Fig. 1

Gene Mutation Provides More Meat on the Hoof. Science, Vol. 227: September 26, 1997
http://www.unc.edu/~wbollenb/beef.html
Later experiments showed it possible to “knockout” the myostatin gene in mice. One can clearly see the stark difference between the normal mouse and “mighty mouse.” (fig.2)
Fig. 2
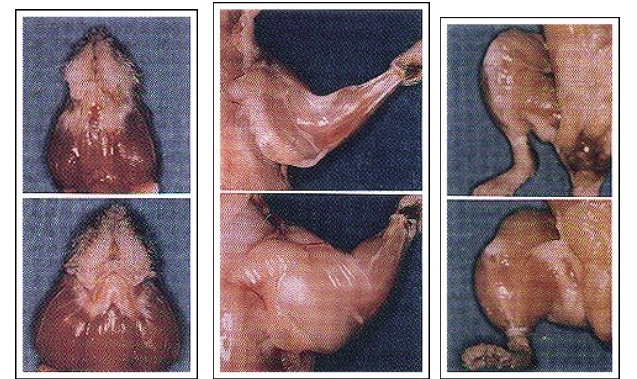
Nature. 1997 May 1;387(6628):83-90.
Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member.
McPherron AC, Lawler AM, Lee SJ.
https://www.ncbi.nlm.nih.gov/pubmed/9139826
Other experiments demonstrate how using DNA for IGF-I over expression also increases muscle cross sectional area in mice. (fig 3) Interestingly enough, this treatment also preserves the fast glycolytic cross sectional area of the aging treated mice, compared to loss of such tissue associated with the aging process in non treated animals. (fig 4)
Fig. 3
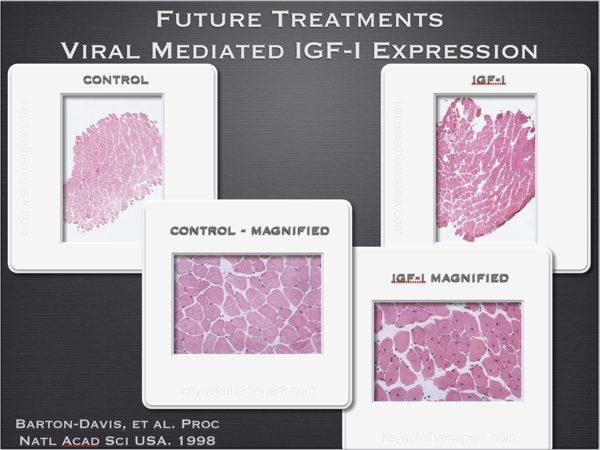
Fig. 4
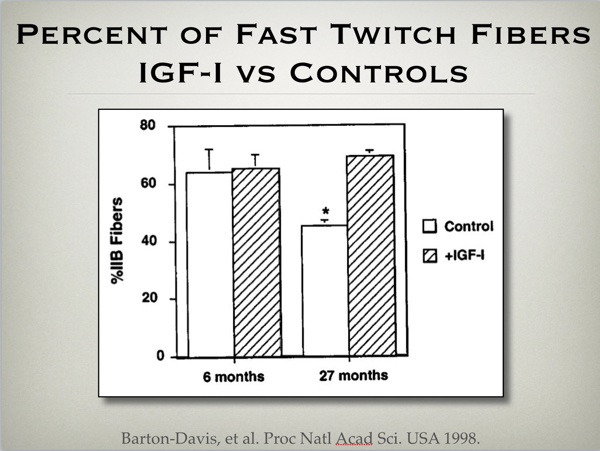
Proc Natl Acad Sci U S A. 1998 Dec 22;95(26):15603-7.
Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function.
Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC28090/
Later, similar mutations were discovered in humans. (figs 5, 6, 7)
Fig. 5
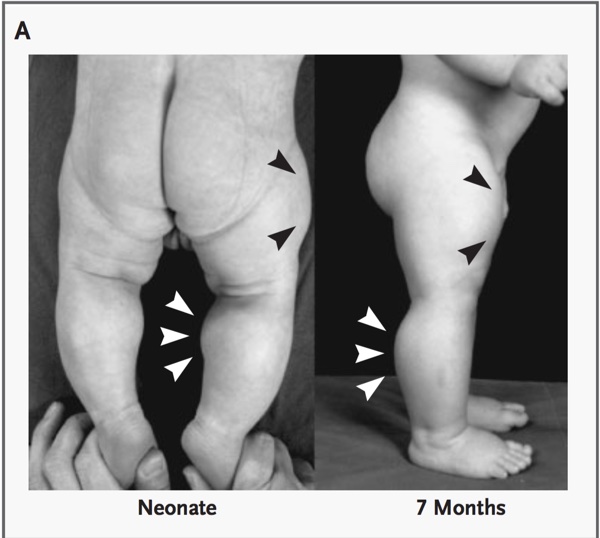
Fig 6 demonstrates an ultrasonograph through the mid-portion of the thigh revealing differences between the subject with the mutation for myostatin deletion and a control infant of the same age and sex. VL denotes vastus lateralis, VI vastus intermedius, VM vastus medialis, RF rectus femoris, and F femur.
Fig. 6
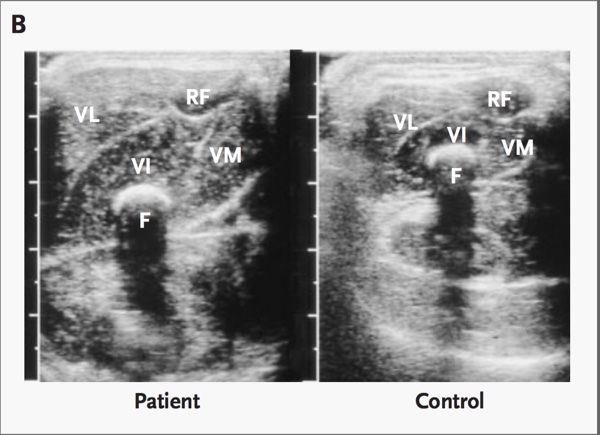
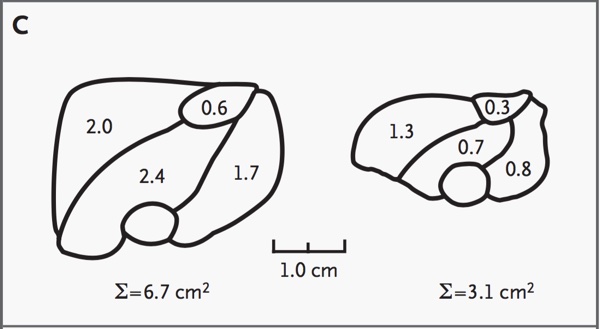
Fig 7 shows a schematic representation of the ultrasonograph in Fig 6, demonstrating the differences in muscle cross-sectional area of the two infants.
Fig. 7
The New England Journal of Medicine
Myostatin Mutation Associated with Gross Muscle Hypertrophy in a Child
Markus Schuelke, M.D., Kathryn R. Wagner, M.D., Ph.D., Leslie E. Stolz, Ph.D., Christoph Hübner, M.D., Thomas Riebel, M.D., Wolfgang Kömen, M.D., Thomas Braun, M.D., Ph.D., James F. Tobin, Ph.D., and Se-Jin Lee, M.D., Ph.D.
http://www.nejm.org/doi/pdf/10.1056/NEJMoa040933
While these mutations are interesting to observe, they are also not trainable. The study of epigenetics shows us that we can increase or decrease the expression of certain genes with an appropriate environmental stimulus. However, we can not change our genotype and thus are genetically limited concerning to results from exercise.
Moreover, there are a number of other genes that have been discovered influencing how an individual adapts to or tolerates an exercise stimulus. These genes are directly linked to one’s muscle fiber type distribution, differences in muscle structural and cytoskeletal proteins, amount of potential binding between myosin and actin contractile proteins, sensitivity to exercise induced micro trauma, inflammatory factors influencing muscle recovery, susceptibility to injury, etc. These factors are trainable. Or rather, I should say once we determine the likely genotype, either through testing or observation, it is possible to develop a training program allowing the individual subject to maximize their genetic potential. One of the things we have been doing differently at Exercise Science, LLC is to attempt, with as much accuracy as possible, to modify the training stimulus to match the clients genetic profile.
Since I wrote the the initial “Exercise and Genetic Variability” lecture in the 2005-2006 timeframe, not only have more genes been discovered influencing one’s ability to adapt to exercise, but we have a deeper understanding of the mechanisms behind the various genotypes. Over the next several months, I will be doing a reboot of the original lecture with more genes, research studies, etc. and how to customize a training program to fit one’s genotype. Moreover, there were several genes and studies I did not include in the original presentation because the data did not display well visually. I am able to further extrapolate the relevance of those genes through the written word, and thus will do so on this blog.
If you have any questions, please leave a comment here or on the Facebook page. We welcome discussions.
Thanks,
Ryan A. Hall
Many of you may be familiar with the Belgium Blue Bull, which was simply bred for muscularity, leading to a mutation for myostatin deletion (fig.1) These bulls were not put on any special training programs, nutritional supplements, creatine monohydrate or hydrochloride, protein powders, anabolic steroids, etc. They simply stand around and graze like any other cow. Myostatin deletion leads to a virtual doubling of the number of muscle fibers when compared to animals without myostatin deletion.
Fig. 1

Gene Mutation Provides More Meat on the Hoof. Science, Vol. 227: September 26, 1997
http://www.unc.edu/~wbollenb/beef.html
Later experiments showed it possible to “knockout” the myostatin gene in mice. One can clearly see the stark difference between the normal mouse and “mighty mouse.” (fig.2)
Fig. 2

Nature. 1997 May 1;387(6628):83-90.
Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member.
McPherron AC, Lawler AM, Lee SJ.
https://www.ncbi.nlm.nih.gov/pubmed/9139826
Other experiments demonstrate how using DNA for IGF-I over expression also increases muscle cross sectional area in mice. (fig 3) Interestingly enough, this treatment also preserves the fast glycolytic cross sectional area of the aging treated mice, compared to loss of such tissue associated with the aging process in non treated animals. (fig 4)
Fig. 3

Fig. 4

Proc Natl Acad Sci U S A. 1998 Dec 22;95(26):15603-7.
Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function.
Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC28090/
Later, similar mutations were discovered in humans. (figs 5, 6, 7)
Fig. 5

Fig 6 demonstrates an ultrasonograph through the mid-portion of the thigh revealing differences between the subject with the mutation for myostatin deletion and a control infant of the same age and sex. VL denotes vastus lateralis, VI vastus intermedius, VM vastus medialis, RF rectus femoris, and F femur.
Fig. 6

Fig 7 shows a schematic representation of the ultrasonograph in Fig 6, demonstrating the differences in muscle cross-sectional area of the two infants.
Fig. 7

The New England Journal of Medicine
Myostatin Mutation Associated with Gross Muscle Hypertrophy in a Child
Markus Schuelke, M.D., Kathryn R. Wagner, M.D., Ph.D., Leslie E. Stolz, Ph.D., Christoph Hübner, M.D., Thomas Riebel, M.D., Wolfgang Kömen, M.D., Thomas Braun, M.D., Ph.D., James F. Tobin, Ph.D., and Se-Jin Lee, M.D., Ph.D.
http://www.nejm.org/doi/pdf/10.1056/NEJMoa040933
While these mutations are interesting to observe, they are also not trainable. The study of epigenetics shows us that we can increase or decrease the expression of certain genes with an appropriate environmental stimulus. However, we can not change our genotype and thus are genetically limited concerning to results from exercise.
Moreover, there are a number of other genes that have been discovered influencing how an individual adapts to or tolerates an exercise stimulus. These genes are directly linked to one’s muscle fiber type distribution, differences in muscle structural and cytoskeletal proteins, amount of potential binding between myosin and actin contractile proteins, sensitivity to exercise induced micro trauma, inflammatory factors influencing muscle recovery, susceptibility to injury, etc. These factors are trainable. Or rather, I should say once we determine the likely genotype, either through testing or observation, it is possible to develop a training program allowing the individual subject to maximize their genetic potential. One of the things we have been doing differently at Exercise Science, LLC is to attempt, with as much accuracy as possible, to modify the training stimulus to match the clients genetic profile.
Since I wrote the the initial “Exercise and Genetic Variability” lecture in the 2005-2006 timeframe, not only have more genes been discovered influencing one’s ability to adapt to exercise, but we have a deeper understanding of the mechanisms behind the various genotypes. Over the next several months, I will be doing a reboot of the original lecture with more genes, research studies, etc. and how to customize a training program to fit one’s genotype. Moreover, there were several genes and studies I did not include in the original presentation because the data did not display well visually. I am able to further extrapolate the relevance of those genes through the written word, and thus will do so on this blog.
If you have any questions, please leave a comment here or on the Facebook page. We welcome discussions.
Thanks,
Ryan A. Hall
blog comments powered by Disqus